Show summary Hide summary
- What we now know about brain waves and paralysis
- How the APL Bioengineering EEG study was designed
- Detailed results: decoding intent to move, step by step
- Connecting EEG to stimulators: toward real motor recovery
- What this means for patients, clinics and policy
- Limitations, open questions and realistic expectations
- How does an EEG brain-computer interface help after paralysis?
- Is this technology a replacement for brain implants?
- Can EEG-based systems restore walking as well as arm movement?
- When might patients see these technologies in routine care?
- Does using a brain-computer interface help the nervous system heal?
What if a simple cap on the head could help bypass a broken spinal cord and restore movement after paralysis, without opening the skull? That is what a new study suggests, showing that Brain Waves recorded from the scalp can reliably reveal when a paralyzed person is trying to move.
This work reshapes how specialists think about Neurotechnology for paralysis: instead of deep brain surgery, future systems may rely on smart algorithms and noninvasive sensors to drive muscles or stimulators in real time.
What we now know about brain waves and paralysis
The latest research, published in APL Bioengineering by AIP Publishing, brings together universities in Italy and Switzerland to test a simple idea. Even when the body is paralyzed, the brain still sends out neural signals for movement; the problem lies in the damaged spinal cord that blocks them.
How Rare Australian Rocks Trace the Birth of a Vital Metal
Bone Cancer Treatment Surprisingly Reduces Tumor Pain
In people with spinal cord injury, the arms and legs often remain structurally intact and the brain functions normally. The disconnect, as also described by brain research from Penn Medicine, happens along the spinal pathway, not in the limb itself. This gap has pushed scientists to look for ways to re-route information around the injury.

From implants to noninvasive brain-computer interfaces
Over the last decade, several groups have shown that implanted electrodes can drive robotic arms or stimulate muscles. A Nature paper in 2016, followed by work on a double neural bypass, proved that signals recorded directly in the brain can help a person with paralysis move an arm and even feel touch again.
Similar implant-based Brain-Computer Interface projects, highlighted by the NIH in its Movement after paralysis reports, have transformed what is considered possible for motor recovery. However, surgery inside the brain carries infection risks, potential long-term scarring and repeated procedures to maintain hardware.
How the APL Bioengineering EEG study was designed
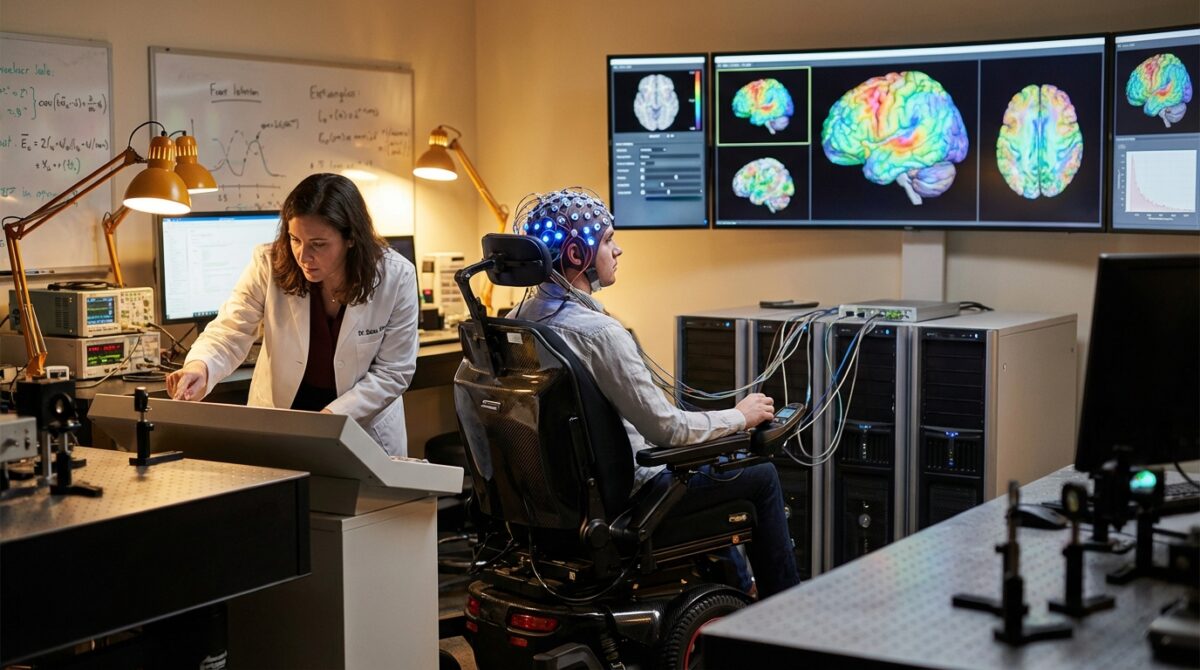
To explore a safer pathway, the Italian–Swiss team tested whether scalp electrodes could decode movement attempts in people with paralysis. The method, led by researcher Laura Toni and colleagues, can be summarized in a single line: record EEG while participants try to move, then use machine learning to classify the resulting activity.
Participants wore an EEG cap containing multiple electrodes while they performed, or attempted to perform, simple movements such as trying to lift a hand or remain still. The study focused on whether algorithms could distinguish “movement attempt” from “no movement” using relatively few trials, a realistic constraint in clinical Neurorehabilitation.
Machine learning meets noisy neural signals
Because EEG electrodes sit on the scalp, they pick up blended activity from millions of neurons and background noise from muscles, blinks and the environment. The team therefore selected a machine learning approach designed for small, complex datasets rather than massive training sets.
They trained the algorithm on short time windows around each cue. The model learned to recognize patterns of oscillations—changes in Brain Waves power in specific frequency bands—that appeared when participants tried to move, even if the limb itself did not budge due to paralysis.
Detailed results: decoding intent to move, step by step
The most important outcome was that the system could reliably tell the difference between “attempted movement” and “rest” using noninvasive EEG alone. This classification is the first building block for a practical Rehabilitation Technology that reacts to a patient’s intent.
The algorithm was less accurate when trying to separate different kinds of movement attempts, such as one gesture versus another. This limitation reflects the fact that signals recorded at the scalp represent a blurred sum of many neural sources, especially for movements controlled by deeper brain regions.
Upper limbs versus lower limbs: why the brain’s map matters
The study confirmed a known principle in Neuroscience: decoding leg movements from EEG is harder than decoding arm movements. The motor areas for the legs lie closer to the brain’s midline, deeper and further from the scalp sensors.
As Toni explained, signals related to hands and arms are located more laterally, giving clearer spatial mapping. Trials like the recent EEG-based work reported by American institutions reach similar conclusions, suggesting that early clinical uses will likely target reaching and grasping before walking.
Connecting EEG to stimulators: toward real motor recovery
The logical next step is to couple this decoding with devices able to stimulate nerves or muscles. Previous clinical experiments, including the brain–body linking microchip case and wearable neural bypass systems, have shown that artificial stimulation can reanimate paralyzed limbs.
In the new EEG-based vision, instead of an implant providing the command signal, a noninvasive Brain-Computer Interface would detect that a person intends to stand or grasp and then trigger a spinal cord stimulator or functional electrical stimulators placed on the skin.
A future noninvasive neurotechnology toolkit
Researchers working on the ReHAB trial and similar studies to restore purposeful movement expect several components to converge in the clinic:
- EEG caps to capture intention from Brain Waves without surgery.
- Smart algorithms to detect specific actions such as standing, stepping or reaching.
- Spinal or peripheral stimulators to activate surviving nerves below the injury.
- Robotic supports and exoskeletons to provide stability and assist complex motions.
- Therapy protocols that combine repeated brain-controlled movement with physical training.
Together, these tools could drive motor recovery by pairing a person’s own neural signals with real, visible movement during Neurorehabilitation.
What this means for patients, clinics and policy
For someone like Marco, a fictional 28-year-old with a cervical spinal cord injury, the difference is tangible. Instead of facing brain surgery, he might enter a trial where a technician fits an EEG cap, calibrates the decoder and connects it to a stimulator during intensive therapy weeks.
Recent coverage of AI-assisted systems, such as trials using artificial intelligence to help paralysis patients move and communicate, shows how quickly algorithms are entering rehabilitation hospitals. The new EEG results suggest that more centers could participate, because the hardware resembles equipment already used for clinical brain monitoring.
Limitations, open questions and realistic expectations
Despite the promise, several constraints remain. The current study involved a relatively small sample and controlled tasks, so performance numbers might look different in busy real-world environments. Signal quality drops with sweat, hair and motion, and long-term daily use has not yet been evaluated.
Most importantly, the findings demonstrate strong correlation between EEG patterns and movement attempts, but not direct causation for recovery of function. Lasting improvements in independence will depend on how well these systems integrate with physical therapy, how reliable they stay over months and how accessible they become in publicly funded health systems.
How does an EEG brain-computer interface help after paralysis?
An EEG-based brain-computer interface records Brain Waves from the scalp while a person attempts to move. Machine learning algorithms detect patterns linked to movement intention. These decoded signals can then be sent to stimulators or robotic devices, helping to activate muscles or assist limbs, creating a new communication route around a damaged spinal cord.
Is this technology a replacement for brain implants?
Noninvasive EEG systems reduce surgical risks and may be suitable for many patients, especially early in rehabilitation. However, implanted devices usually provide stronger, more specific neural signals. Current evidence suggests EEG may complement, rather than fully replace, implant-based neurotechnology, with different tools matched to different clinical needs.
Can EEG-based systems restore walking as well as arm movement?
Decoding leg movements from the scalp is more challenging because the relevant brain areas lie deeper and closer to the midline. Studies indicate that upper limb control is currently more accurate. Walking assistance using EEG is an active research area, often combined with exoskeletons and spinal cord stimulation, but performance still lags behind arm-focused applications.
When might patients see these technologies in routine care?
Some implant-based systems and spinal stimulators are already in highly specialized centers, and early neurorehabilitation trials are underway. Widespread use of noninvasive EEG brain-computer interfaces will require larger clinical studies, regulatory approvals and clear reimbursement strategies, which may take several years, depending on country and health system.
Does using a brain-computer interface help the nervous system heal?
Ancient Giant Kangaroos Were Capable of Hopping After All
Stopping HIV in Its Tracks: The Century’s Most Innovative Breakthroughs
Current data mainly show that these systems can enable functional movement by bypassing injured pathways. There are early indications that pairing intention with assisted movement might promote plasticity and partial recovery, but evidence is still limited. Researchers therefore speak of supporting function rather than guaranteeing biological repair of the spinal cord.